High-Performance Liquid Chromatography
Principles, Methods, and Practical Applications
High-performance liquid Chromatography (HPLC) is an advanced analytical technique used to separate, identify, and quantify components in a mixture. It operates by pumping a liquid mobile phase at high pressure through a column packed with a stationary phase, separating compounds based on their interactions with the stationary phase. HPLC is widely used in pharmaceuticals, environmental analysis, food testing, and biochemistry due to its accuracy, sensitivity, and versatility.
Functions of High-Performance Liquid Chromatography:
- Mobile Phase: A liquid solvent (or mixture of solvents) is pumped at high pressure through the system.
- Sample Injection: The sample is injected into the mobile phase stream, which carries it into the column.
- Stationary Phase: The column is packed with tiny particles coated with a stationary phase, which interacts differently with each component in the sample.
- Separation: As the sample moves through the column, components separate based on their affinity for the stationary phase, some interact more strongly and move slower, while others move faster.
- Detection: Separated components pass through a detector (e.g., UV-Vis, fluorescence, or mass spectrometer), which generates signals used to identify and quantify each component.
- Data Analysis: The detector’s output is recorded as a chromatogram, showing peaks corresponding to each component.
Uses of High-Performance Liquid Chromatography:

- High Precision: It provides accurate and reproducible results for complex mixtures.
- Sensitivity: It detects and quantifies trace amounts of compounds.
- Versatility: It can analyze a wide range of compounds, from small molecules to large biomolecules.
- Speed: It delivers fast and efficient separations compared to traditional methods.
- Automation: It is Easily integrated with automated systems for high-throughput analysis.
Examples of Applications:
- Pharmaceuticals: Analyzing drug purity, stability, and potency, or quantifying active pharmaceutical ingredients (APIs).
- Environmental Testing: Detecting pollutants, pesticides, or contaminants in water, soil, or air samples.
- Food and Beverage: Testing for additives, preservatives, or contaminants in food products.
- Clinical Research: Measuring metabolites, hormones, or drugs in biological samples like blood or urine.
- Biochemistry: Separating and identifying proteins, peptides, or nucleic acids.
HPLC is a cornerstone of modern analytical chemistry, offering unmatched precision, sensitivity, and efficiency for a wide range of applications in science and industry.
Principles, Methods, and Practical Applications
High-performance liquid Chromatography (HPLC) is a widely used analytical technique in chemistry for the separation, identification, and quantification of components in a mixture. It is highly effective for analyzing compounds that are non-volatile or thermally unstable. HPLC plays a crucial role in various industries, including pharmaceuticals, environmental testing, food and beverage, and forensic science. This article explores the principles, methods, and practical applications of HPLC chromatography.
What is High-Performance Liquid Chromatography (HPLC)?
High-Performance Liquid Chromatography (HPLC) is a form of liquid chromatography used to separate components of a mixture based on their interactions with a stationary phase and a liquid mobile phase under high pressure. The technique is highly accurate, efficient, and suitable for both qualitative and quantitative analysis of complex mixtures.
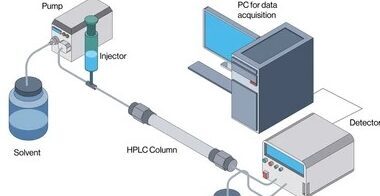
The main components of an HPLC system include:
- Mobile Phase – A liquid solvent that carries the sample through the column.
- Stationary Phase – A solid material, usually silica-based, packed inside the column.
- Pump – Provides high pressure to push the mobile phase through the column.
- Injector – Introduces the liquid sample into the mobile phase.
- Column – A tube packed with the stationary phase where separation occurs.
- Detector – Identifies and measures the separated components.
- Data System – Records and analyzes the data from the detector.
Principles of HPLC

The principle of HPLC is based on the separation of components in a mixture due to their different interactions with the stationary phase and mobile phase. When the sample is injected, the components move through the column at different rates based on their affinity for the stationary phase.
The factors influencing separation in HPLC are:
- Polarity of Components: Components with higher polarity will interact more with a polar stationary phase and elute slower.
- Mobile Phase Composition: Changing the composition of the mobile phase affects the separation efficiency.
- Column Temperature: Temperature can impact the interaction strength between components and the stationary phase.
- Flow Rate: Higher flow rates reduce analysis time but may affect resolution.
Types of High-Performance Liquid Chromatography
HPLC can be classified into different types based on the mode of separation and the nature of the stationary phase.
1. Normal Phase HPLC
- Uses a polar stationary phase (e.g., silica) and a non-polar mobile phase.
- Separates compounds based on their polarity.
- Polar compounds elute slower as they interact more with the stationary phase.
2. Reverse Phase HPLC (RP-HPLC)
- Uses a non-polar stationary phase (e.g., C18 silica) and a polar mobile phase.
- Non-polar compounds elute slower as they interact more with the stationary phase.
- Widely used for pharmaceutical, food, and environmental analysis.
3. Size Exclusion Chromatography (SEC)
- Separates compounds based on their molecular size.
- Larger molecules elute faster as they do not penetrate the porous stationary phase.
- Commonly used for protein and polymer analysis.
4. Ion Exchange Chromatography (IEC)
- Separates ionic compounds based on their charge.
- Uses a charged stationary phase to attract oppositely charged compounds.
- Commonly used for water analysis and protein purification.
Materials Required for HPLC
To perform HPLC, the following materials are required:
- HPLC instrument (pump, injector, column, detector)
- Mobile phase solvents (e.g., methanol, acetonitrile, water)
- Stationary phase (silica-based or C18 column)
- Sample solution
- Data analysis software
Steps to Perform HPLC
Step 1: Sample Preparation
- Dissolve the sample in a suitable solvent.
- Filter the sample to remove any particulate matter.
Step 2: Sample Injection
- Inject a small volume of the sample (typically 10-20 µL) into the injector.
- The injector introduces the sample into the mobile phase.
Step 3: Separation in the Column
- The mobile phase carries the sample through the column.
- Components interact differently with the stationary phase, causing separation.
Step 4: Detection
- The separated components pass through the detector.
- The detector generates a signal proportional to the concentration of each component.
Step 5: Data Analysis
- The output is displayed as a chromatogram.
- Each peak represents a different component.
- The area under each peak quantifies the concentration of the component.
Practical Applications of High-Performance Liquid Chromatography
HPLC is widely used in various fields for analytical purposes.
1. Pharmaceutical Industry
- Quality control of pharmaceutical products.
- Determination of drug purity and content.
- Analysis of active pharmaceutical ingredients (APIs).
2. Environmental Testing
- Detection of contaminants in water, soil, and air.
- Monitoring pollution levels.
3. Food and Beverage Industry
- Detection of food additives, preservatives, and contaminants.
- Analysis of vitamins, amino acids, and organic acids.
4. Clinical and Biomedical Research

- Analysis of blood, urine, and plasma samples.
- Monitoring therapeutic drug levels.
5. Chemical and Petrochemical Industry
- Analysis of raw materials and final products.
- Quality control in manufacturing.
Advantages of High-Performance Liquid Chromatography
- High accuracy, precision, and sensitivity.
- Can analyze complex mixtures with high resolution.
- Rapid and efficient separation process.
- Can handle both volatile and non-volatile compounds.
Limitations of High-Performance Liquid Chromatography
- High operating cost due to expensive equipment and solvents.
- Requires skilled personnel for operation and data analysis.
- Time-consuming sample preparation.
High-Performance Liquid Chromatography (HPLC) is an essential analytical technique widely used in various fields, including pharmaceuticals, food, environmental testing, and biomedical research. Its ability to provide accurate and high-resolution analysis makes it indispensable for qualitative and quantitative analysis of complex mixtures. Understanding its principles, methods, and practical applications enables scientists and researchers to effectively utilize HPLC for advanced chemical analysis.
What is HPLC and its principle?
What is High-Performance Liquid chromatography used for?
Sensitivity: Detects and quantifies trace amounts of compounds.
Versatility: It can analyze a wide range of compounds, from small molecules to large biomolecules.
Speed: It delivers fast and efficient separations compared to traditional methods.
Automation: It is Easily integrated with automated systems for high-throughput analysis.
What are the advantages of High-Performance Liquid chromatography?
Sensitivity: It Detects and quantifies trace amounts of compounds.
Versatility: It Can analyze a wide range of compounds, from small molecules to large biomolecules.
Speed: It delivers fast and efficient separations compared to traditional methods.
Automation: It is Easily integrated with automated systems for high-throughput analysis.